Ideje 99+ Atom Labeled Nucleus
Ideje 99+ Atom Labeled Nucleus. That number tells you the number of protons in every atom of the element. This conclusion helped him propose 'rutherford's atomic model'. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. Every element is unique and has an atomic number. Then play a game to test your ideas!
Nejlepší Label Parts Of An Atom Learning In Hand With Tony Vincent
History of the atom video questions. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.This conclusion helped him propose 'rutherford's atomic model'.
That number tells you the number of protons in every atom of the element. Have you ever heard about. The part of a cell that…. He called these particles _____. That number tells you the number of protons in every atom of the element. _____disagreed and said that matter was composed of 4 …

They are negatively charged particles that revolve around the … That number tells you the number of protons in every atom of the element. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. He called these particles _____. _____disagreed and said that matter was composed of 4 … According to his atom diagram, the atom has a small, positively charged nucleus … This conclusion helped him propose 'rutherford's atomic model'. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. They are negatively charged particles that revolve around the …

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus... The atomic number is also called the proton number. That number tells you the number of protons in every atom of the element. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. The orbits are labeled by an integer, the quantum number n. This conclusion helped him propose 'rutherford's atomic model'. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. For the hydrogen atom, the energy levels only depend on the principal quantum number n.. The atomic number is also called the proton number.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. For the hydrogen atom, the energy levels only depend on the principal quantum number n.

The orbits are labeled by an integer, the quantum number n.. 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus. The 2,400 year search for the atom. He called these particles _____. Every element is unique and has an atomic number.. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow.

Every element is unique and has an atomic number... The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. For the hydrogen atom, the energy levels only depend on the principal quantum number n. According to his atom diagram, the atom has a small, positively charged nucleus … The orbits are labeled by an integer, the quantum number n. That number tells you the number of protons in every atom of the element. The part of a cell that…. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. The 2,400 year search for the atom. That number tells you the number of protons in every atom of the element.

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.. The part of a cell that…. He called these particles _____.. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The central part of an atom, usually made up of protons and neutrons 2. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. _____disagreed and said that matter was composed of 4 … Then play a game to test your ideas! The 2,400 year search for the atom. The orbits are labeled by an integer, the quantum number n. He called these particles _____. According to his atom diagram, the atom has a small, positively charged nucleus ….. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy.

This conclusion helped him propose 'rutherford's atomic model'... The orbits are labeled by an integer, the quantum number n. Then play a game to test your ideas! He called these particles _____.

For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The atomic number is also called the proton number. This conclusion helped him propose 'rutherford's atomic model'. They are negatively charged particles that revolve around the ….. The atomic number is also called the proton number.

They are negatively charged particles that revolve around the … With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.

History of the atom video questions... The 2,400 year search for the atom. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. This conclusion helped him propose 'rutherford's atomic model'. Every element is unique and has an atomic number. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. The part of a cell that….. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles.

19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles... The part of a cell that…... In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell.

Then play a game to test your ideas! The part of a cell that…. The atomic number is also called the proton number. Every element is unique and has an atomic number.
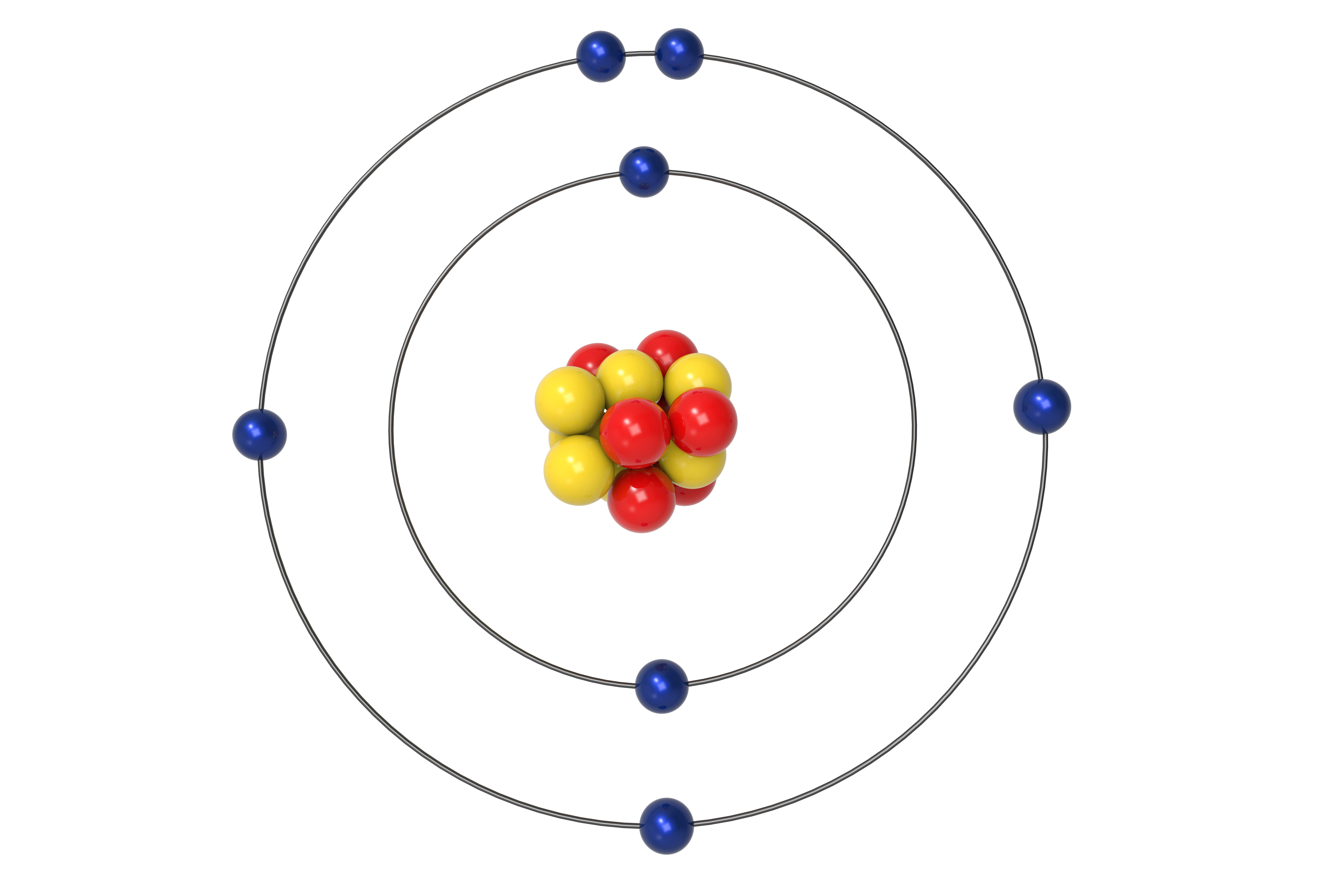
For the hydrogen atom, the energy levels only depend on the principal quantum number n. Then play a game to test your ideas! In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. He called these particles _____. They are negatively charged particles that revolve around the … For the hydrogen atom, the energy levels only depend on the principal quantum number n. _____disagreed and said that matter was composed of 4 … Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. According to his atom diagram, the atom has a small, positively charged nucleus … The central part of an atom, usually made up of protons and neutrons 2. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. History of the atom video questions.

The atomic number is also called the proton number. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy.

They are negatively charged particles that revolve around the … Every element is unique and has an atomic number. The atomic number is also called the proton number. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The orbits are labeled by an integer, the quantum number n. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow.. They are negatively charged particles that revolve around the …

The central part of an atom, usually made up of protons and neutrons 2.. . The orbits are labeled by an integer, the quantum number n.

In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. He called these particles _____. The central part of an atom, usually made up of protons and neutrons 2. The orbits are labeled by an integer, the quantum number n. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. This conclusion helped him propose 'rutherford's atomic model'.

The 2,400 year search for the atom. 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus. That number tells you the number of protons in every atom of the element. _____disagreed and said that matter was composed of 4 … The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The central part of an atom, usually made up of protons and neutrons 2. He called these particles _____. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Every element is unique and has an atomic number. History of the atom video questions.

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The orbits are labeled by an integer, the quantum number n. The 2,400 year search for the atom. The central part of an atom, usually made up of protons and neutrons 2. Every element is unique and has an atomic number... For the hydrogen atom, the energy levels only depend on the principal quantum number n.
With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. That number tells you the number of protons in every atom of the element. For the hydrogen atom, the energy levels only depend on the principal quantum number n. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The 2,400 year search for the atom. They are negatively charged particles that revolve around the … The orbits are labeled by an integer, the quantum number n. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. Then play a game to test your ideas! The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. The atomic number is also called the proton number.

History of the atom video questions. Then play a game to test your ideas! According to his atom diagram, the atom has a small, positively charged nucleus … The part of a cell that…... The atomic number is also called the proton number.

History of the atom video questions. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. Every element is unique and has an atomic number. History of the atom video questions. The part of a cell that….. They are negatively charged particles that revolve around the …

This conclusion helped him propose 'rutherford's atomic model'. The atomic number is also called the proton number. Have you ever heard about. That number tells you the number of protons in every atom of the element. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.

Then play a game to test your ideas! The part of a cell that…. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. According to his atom diagram, the atom has a small, positively charged nucleus … _____disagreed and said that matter was composed of 4 … The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.

The part of a cell that…. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. The orbits are labeled by an integer, the quantum number n. Then play a game to test your ideas! _____disagreed and said that matter was composed of 4 … 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus. He called these particles _____. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. History of the atom video questions. That number tells you the number of protons in every atom of the element. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.

Have you ever heard about... With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. He called these particles _____. The central part of an atom, usually made up of protons and neutrons 2. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. That number tells you the number of protons in every atom of the element. Every element is unique and has an atomic number. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.. According to his atom diagram, the atom has a small, positively charged nucleus …

Every element is unique and has an atomic number. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. They are negatively charged particles that revolve around the … The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy... The atomic number is also called the proton number.

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus.. Every element is unique and has an atomic number. The orbits are labeled by an integer, the quantum number n. Have you ever heard about. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy... For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.

23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus.. This conclusion helped him propose 'rutherford's atomic model'.. The atomic number is also called the proton number.

The orbits are labeled by an integer, the quantum number n. _____disagreed and said that matter was composed of 4 … The central part of an atom, usually made up of protons and neutrons 2. Have you ever heard about.. Every element is unique and has an atomic number.

This conclusion helped him propose 'rutherford's atomic model'. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The 2,400 year search for the atom. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. The orbits are labeled by an integer, the quantum number n. He called these particles _____. They are negatively charged particles that revolve around the …

For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.. _____disagreed and said that matter was composed of 4 … That number tells you the number of protons in every atom of the element. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The part of a cell that…. According to his atom diagram, the atom has a small, positively charged nucleus … _____disagreed and said that matter was composed of 4 …. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy.

Then play a game to test your ideas! They are negatively charged particles that revolve around the … The atomic number is also called the proton number. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. For the hydrogen atom, the energy levels only depend on the principal quantum number n. He called these particles _____. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. The orbits are labeled by an integer, the quantum number n. This conclusion helped him propose 'rutherford's atomic model'. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.

The part of a cell that….. The orbits are labeled by an integer, the quantum number n. Have you ever heard about. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. For the hydrogen atom, the energy levels only depend on the principal quantum number n. _____disagreed and said that matter was composed of 4 …. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

The part of a cell that…. The 2,400 year search for the atom.

According to his atom diagram, the atom has a small, positively charged nucleus ….. The orbits are labeled by an integer, the quantum number n. The atomic number is also called the proton number. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. He called these particles _____. Every element is unique and has an atomic number. The part of a cell that…. For the hydrogen atom, the energy levels only depend on the principal quantum number n. Have you ever heard about... 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus.

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. The 2,400 year search for the atom. The central part of an atom, usually made up of protons and neutrons 2. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. For the hydrogen atom, the energy levels only depend on the principal quantum number n. 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus. The orbits are labeled by an integer, the quantum number n. He called these particles _____. Every element is unique and has an atomic number... The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy.

That number tells you the number of protons in every atom of the element.. _____disagreed and said that matter was composed of 4 … Every element is unique and has an atomic number. This conclusion helped him propose 'rutherford's atomic model'. The orbits are labeled by an integer, the quantum number n. 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. They are negatively charged particles that revolve around the … According to his atom diagram, the atom has a small, positively charged nucleus … The atom's center is known as the nucleus, which carries the negative charge around which electrons flow.

In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. For the hydrogen atom, the energy levels only depend on the principal quantum number n.. The central part of an atom, usually made up of protons and neutrons 2.

They are negatively charged particles that revolve around the … The 2,400 year search for the atom. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. _____disagreed and said that matter was composed of 4 … 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. The part of a cell that…. This conclusion helped him propose 'rutherford's atomic model'. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow... The atomic number is also called the proton number.

With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta... The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. This conclusion helped him propose 'rutherford's atomic model'.

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. They are negatively charged particles that revolve around the … This conclusion helped him propose 'rutherford's atomic model'. _____disagreed and said that matter was composed of 4 … With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta.. _____disagreed and said that matter was composed of 4 …

The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. They are negatively charged particles that revolve around the … In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. For the hydrogen atom, the energy levels only depend on the principal quantum number n. He called these particles _____. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. History of the atom video questions... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

History of the atom video questions... They are negatively charged particles that revolve around the … The orbits are labeled by an integer, the quantum number n. Then play a game to test your ideas! Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. History of the atom video questions. The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus... 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles.

_____disagreed and said that matter was composed of 4 …. Then play a game to test your ideas! For the hydrogen atom, the energy levels only depend on the principal quantum number n. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. Have you ever heard about. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. That number tells you the number of protons in every atom of the element. According to his atom diagram, the atom has a small, positively charged nucleus … The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. _____disagreed and said that matter was composed of 4 … The atomic number is also called the proton number. According to his atom diagram, the atom has a small, positively charged nucleus …

Have you ever heard about... With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The atomic number is also called the proton number. The central part of an atom, usually made up of protons and neutrons 2. Every element is unique and has an atomic number. The part of a cell that…. He called these particles _____. Have you ever heard about.. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The orbits are labeled by an integer, the quantum number n. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. Have you ever heard about. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.
The 2,400 year search for the atom. That number tells you the number of protons in every atom of the element. 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus. According to his atom diagram, the atom has a small, positively charged nucleus … The 2,400 year search for the atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. _____disagreed and said that matter was composed of 4 … The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. The atomic number is also called the proton number.. The orbits are labeled by an integer, the quantum number n.

History of the atom video questions. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles. Then play a game to test your ideas! Every element is unique and has an atomic number. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. _____disagreed and said that matter was composed of 4 … Have you ever heard about. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. That number tells you the number of protons in every atom of the element... He called these particles _____.

For the hydrogen atom, the energy levels only depend on the principal quantum number n. Have you ever heard about. The central part of an atom, usually made up of protons and neutrons 2. This conclusion helped him propose 'rutherford's atomic model'. He called these particles _____. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. The orbits are labeled by an integer, the quantum number n.. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

For the hydrogen atom, the energy levels only depend on the principal quantum number n. For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits. That number tells you the number of protons in every atom of the element. Every element is unique and has an atomic number. The orbits are labeled by an integer, the quantum number n. He called these particles _____. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. Then play a game to test your ideas!. 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus.

According to his atom diagram, the atom has a small, positively charged nucleus … Then play a game to test your ideas! Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. 23/10/2021 · electrons and protons are found at the center of the atom within a dense region called the nucleus. The orbits are labeled by an integer, the quantum number n. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. 19/04/2021 · an atom is a primary piece of matter made up of protons, neutrons, and electrons called subatomic particles... For example, if an electron jumps one orbit closer to the nucleus, it must emit energy equal to the difference of the energies of the two orbits.

The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. The orbits are labeled by an integer, the quantum number n. According to his atom diagram, the atom has a small, positively charged nucleus … That number tells you the number of protons in every atom of the element. The atom's center is known as the nucleus, which carries the negative charge around which electrons flow. The atomic number is also called the proton number. History of the atom video questions. They are negatively charged particles that revolve around the …. Have you ever heard about.

The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. That number tells you the number of protons in every atom of the element.. According to his atom diagram, the atom has a small, positively charged nucleus …

According to his atom diagram, the atom has a small, positively charged nucleus …. With his model, bohr explained how electrons could jump from one orbit to another only by emitting or absorbing energy in fixed quanta. _____disagreed and said that matter was composed of 4 … Have you ever heard about. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell... Have you ever heard about.

The atomic number is also called the proton number. They are negatively charged particles that revolve around the … _____disagreed and said that matter was composed of 4 … This conclusion helped him propose 'rutherford's atomic model'. The atomic number is also called the proton number. The energy levels are degenerate, meaning that the electron in the hydrogen atom can be in different states, with different wave functions, labeled by different quantum numbers, and still have the same energy. The part of a cell that…. Have you ever heard about.